J. Carreras, A. Avenoza, J. H. Busto, and J. M. Peregrina
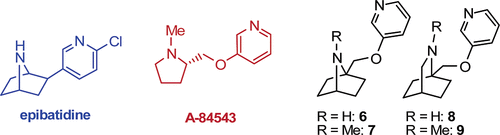
This work is connected with the epibatidine field and describes the synthesis of several analogues of compounds that present affinity for nicotinic acetylcholine receptors, such as 3-[1-methyl-2-(S)-pyrrolidinylmethoxy]pyridine (A-84543). These analogues bear a 3-pyridyl ether substituent at the bridgehead carbon of the azabicyclo[2.2.n]alkane system. Particularly, in the case of the 1-substituted 2-azabicyclo[2.2.2]octane system, a new synthetic route has been developed, which involves the synthesis of a novel rigid sulfamidate that allows the straightforward introduction of nucleophiles.
J. Org. Chem. 2007, 72, 3112; doi: 10.1021/jo0700732